☀️The Voyage of a Photon: From Sun to Sleep
Follow a photon from the stars down to your brain and learn about how this influences human sleep, health, and mental wellbeing.
Human civilization has long been guided by the stars. From early astronomers creating legends to explain the small white dots that appear in the night sky to our current fanaticism of exploring what lies beyond our atmosphere, humanity has been enthralled by this most ancient light display.
Perhaps a fitting motto for our species would be ad astra per aspera. However, a star, which is often taken for granted, has had a more subtle, yet no less profound, impact upon the human condition - our sun. This sphere of hydrogen and helium has single-handedly shaped life on earth and would take an entire book to fully explain. But this article shall focus on what is perhaps less considered: the impact of the sun on the circadian rhythms of the human body.
A circadian rhythm is an internal body clock that regulates processes such as sleep and eating habits. The term originates from the Latin phrase ‘circa diem’, which means ‘about a day’.
In particular, this article will follow the path of a photon all the way from the sun, or one of those stars that are lightyears away, to the human eye and ultimately the rest of the body. Through this journey, it shall become clear that light has a profound impact on regulating our circadian rhythms. Importantly, this will show how imbalances in the mechanisms behind these clocks can lead to disease states such as insomnia, Alzheimer’s and metabolic conditions.
Let's embark upon the voyage of a photon.
Disembarking at the Eye
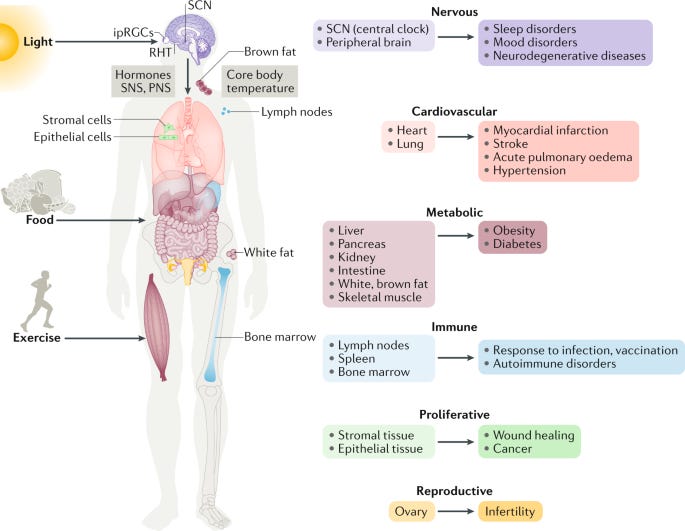
Once the photon has crossed intra or interstellar space, it will enter the eye. One imagines this as the final harbour for lost particles; a great port bustling with all sorts of electronic signals, towering cranes of protein and photons arriving at customs en masse. The arrival of these photons causes the activation of intrinsically photosensitive retinal ganglion cells (ipRGCs), which are a type of cell found on the retina. However, unlike the rod and cone cells in the eye, ipRGCs have no role in vision- they are mere light sensors. In fact, these cells tend to remain functional in blind people, which explains why their circadian rhythms are undisturbed by loss of visual cues.
ipRGCs are excited by the activation of melanopsin photopigments. When a photon hits this molecule, it provides energy, which causes it to change shape and become ‘turned on’. It is then able to activate receptors that initiate a small electrical impulse in the cell. Ultimately, many of these electrical impulses will come together to activate the retinohypothalamic tract (RHT) (this is just a sophisticated way of saying a nerve that travels from the retina to the hypothalamus, which is a structure in the brain). Think of this as a motorway that goes to the brain. However, the photons can’t get a visa to access it. Instead, they pass on the message to electrons, which act as couriers, and drive to the brain in their place.
Arriving in the Brain
The electrons have speedy transport and are soon within sight of the metropolis, the capital city of the body. This particular road (the RHT) leads the electrons to the suprachiasmatic nuclei (SCN) (another structure in the brain), which has been discovered to act as the central circadian pacemaker (perhaps Big Ben might be an apt analogy). In fact, light causes up to 40% of SCN neurons to change their excitability. Although this pacemaker can act independently of the environment, it is beneficial to calibrate it ever so often to ensure that the body’s time is an accurate reflection of real time (i.e. day or night).
The SCN has several roles, one of which is to stimulate the release of melatonin, which shall be returned to later in the article. However, its main purpose is to send messages to the circadian clocks located throughout the body. This is an important concept to understand. The body doesn’t just have one clock but many throughout different organs and even specific tissues. This means that different clocks tick at different rates to reflect the needs of the tissue or organ that it is located in.
Therefore, think of the SCN as a master clock that calibrates other peripheral clocks based on the environmental information provided by the humble photon still caught up with border security at customs. Therefore, our electron couriers are now tasked with relaying their message to tissues around the body. But now, in addition to the rapid electrons, the SCN can dispatch hormones that travel in the slow trucks of the vascular system, as well as causing alterations in body temperature to alter peripheral clocks. As the old saying goes, all roads lead to the suprachiasmatic nuclei (but only as far as circadian rhythms are concerned).
Arriving in the Peripheral Tissues
The electronic and hormonal messengers now arrive in the provincial towns of the body, where life is a little simpler and quieter than the capital. Here they will alter the function of the molecules that constitute the clock, which so far have not been discussed.
**The circadian clocks function via proteins that inhibit their own production**. This can be thought of as a factory that produces robots with a limited number of cogs. The more robots it produces the slower the production becomes as the factory runs low on cogs. The factory then has to wait until the robots break and can be recycled to replenish its stock of cogs.
A similar process happens in the body. It produces proteins via transcription and translation of DNA and then has to wait until the proteins are degraded by processes such as phosphorylation before more can be produced. This ‘waiting’ provides the rhythm of the clock.
The full molecular complexity here is omitted. The take home message is that there are a series of molecules which undergo regular sequences of synthesis and degradation.
Thus, altering the rate at which the proteins degrade (by activation or inhibition of other proteins in the cell) leads to a slower or faster clock.
This, in essence, is what the effect of the SCN is on peripheral tissues, a tweaking of the cellular environment to calibrate peripheral tissues with the external environment and especially light.
These clocks have several important local effects, such as the control of glucose homeostasis by the liver and pancreatic clocks, as well as ovulation by the ovarian clock. It has also been demonstrated that normal activity levels and body weight are achieved by having a functioning circadian clock in muscle tissue.
This leads to the questions of what happens when circadian rhythms fall out of sync.
Effects of Circadian Clocks on Disease
There are several mechanisms by which circadian dysfunction can result in disease. However, this article focuses on melatonin as this pathway is dependent on the photons that initiated this saga.
To understand the mechanism we return to the SCN district of the capital. As has already been alluded to, the SCN is responsible for the regulation of melatonin release.
Although the exact pathway is thought to occur due to the inhibition of melatonin synthetic enzymes like N-acetyltransferase in the pineal gland, the overview is that melatonin production is reduced by light.
Importantly, it has been discovered that the SCN is more sensitive to short or blue wavelength light, which may explain why looking at phone screens before bed reduces sleep quality and duration.
In fact, circadian rhythm disorders include various sleep disorders (e.g. delayed sleep phase disorder, jet lag, shift work disorder). These disorders include several notable symptoms such as insomnia, excessive daytime sleepiness, difficulty waking up in the morning, sleep loss, depression, poor work or school performance, and inability to meet social obligations.
In addition to these neurological disorders, melatonin dysfunction has also been associated with diseases such as Alzheimer's, Parkinson's disease, dermatologic, psychiatric, cardiovascular, and numerous cancers.
Consequently, it is clear that maintaining circadian rhythms is fundamental to good health, in which the seemingly humble photon may play an oversized role.
To be clear, this article is not suggesting that using electronic devices before going to sleep will cause these conditions. However, such behaviour is a risk factor. Consequently, it is advisable to rethink personal routines to include ‘screenless’ time before sleep. This could include reading a book, having a conversation with flatmates or partners, or simply preparing for the next day.
Journey’s End
This article has recounted the journey of a particle that many are unlikely to consider in everyday life but consistently plays a role of singular importance in human health. The readers of this article should possess the understanding of how light leads to the circadian rhythms that regulate the patterns of our life. In addition, it should now become apparent that poor sleep hygiene and errors in the body’s circadian clocks can lead to various pathologies. The ultimate conclusion of this article is to pursue healthier night time routines in order to boost personal health and wellbeing as a form of preventive healthcare.
So next time you look out into the stars, consider the photons that are streaming into your eyes and the effect that they will have throughout your body. We are all connected, we are within a singular universe - not above it, we are mere mortals. Ad astra per aspera!